Prices incl. VAT plus shipping costs
In stock, delivery time approx. 1-2 days**
- Order number: OF11607BPIP1
- EAN 4251496612260
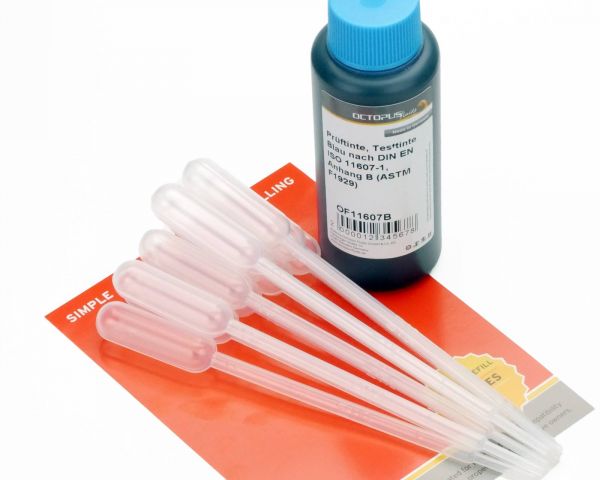
This blue test ink complies with the current DIN EN ISO 11607-1, Appendix B (American ASTM F1929) for testing packaging for medical devices to be sterilized in the final packaging. The test ink is available in stable HDPE plastic bottles with 10 pipettes. The ink has a minimum shelf life of 2 years. This test ink is also available in other colours on request and with the corresponding purchase quantity.
DIN EN ISO 11607-1:2017: “Packaging for terminally sterilized medical devices - Part 1: Requirements for materials, sterile barrier systems and packaging systems”
Note: This standard applies to partially porous sealing materials (paper-film bags). If non-porous film material is used, the test ink according to ASTM F3039 is required.
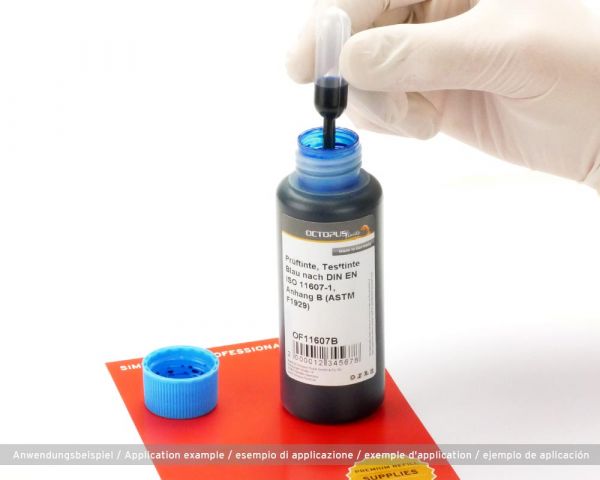
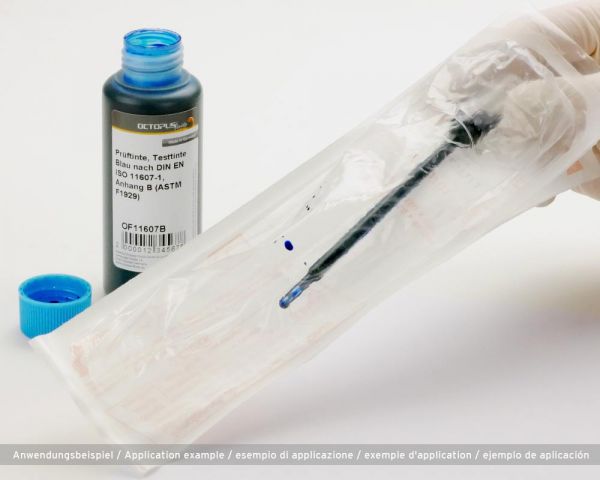
DIN EN ISO 11607-1, Appendix B (ASTM F1929) specifies a test procedure that can be used to determine whether sealing seams of medical packaging seal sterilely. Also known as seal tightness test. During the functional test of sealed seams of medical packaging, this ink is used to check the seam tightness. If the ink leaks, it can be assumed that the medical device is not sterile and the packaging process should be adapted.
These test inks according to DIN EN ISO 11607-1, Appendix B (ASTM F1929) are manufactured by Octopus Fluids GmbH & Co. KG and their physical properties are adjusted according to DIN EN ISO 11607-1, Appendix B.
When testing sealing seams, our test ink makes constant and reliable statements thanks to the consistent quality of our batch test. The ink is also available in further colours. For this you may make your inquiry without obligation.
The DIN EN ISO 11607-1, Annex B is to be obtained through: Beuth Verlag GmbH, Sales of foreign standards, 10772 Berlin (Address: Burggrafenstr. 6, 10787 Berlin).
 Transfer pipettes with dosage tip and plastic bulb, vol. 1 ml
Transfer pipettes with dosage tip and plastic bulb, vol. 1 ml
 Test ink blue according to norm ASTM F3039 with pipettes
Test ink blue according to norm ASTM F3039 with pipettes







 Manufacturer info
Manufacturer info